Introduction
Combination treatment with intravenous immunoglobulin (IVIG) and aspirin is the standard first-line treatment for acute Kawasaki disease (KD) [1,2]. However, approximately 10%–20% of KD patients are resistant to initial IVIG treatment. The American Heart Association (AHA) guidelines [1] define refractory KD as persistent or recrudescent fever at least 36 hours after the completion of the first IVIG dose. However, some studies have used 24 or 48 hours as the cut-off, and the term “resistant KD” is sometimes used interchangeably with “refractory KD” [3–6].
Patients with KD who are resistant to initial IVIG are at increased risk for developing coronary artery lesions (CALs), including aneurysms. Numerous studies have demonstrated that non-responders to initial IVIG therapy are at a higher risk of developing CALs compared to responders. For instance, Tremoulet et al. [2] analyzed 362 KD patients and found that coronary aneurysms developed in 9 of 60 (15%) IVIG-resistant patients compared with 9 of 302 (3%) IVIG-responsive patients (P = 0.0008). IVIG resistance was strongly associated with an increased rate of coronary aneurysms. Infliximab has been used both in combination with IVIG as an intensification of initial treatment and as a second-line option for refractory KD. However, no consensus exists regarding the optimal treatment for high-risk refractory KD patients.
This review aims to provide the usefulness of infliximab in patients with KD (both first-line and second-line treatment) and to investigate the optimal dosing and potential side effects of infliximab.
Main Subject
A literature search was conducted using the PubMed database to identify studies on infliximab in KD, using the terms “Kawasaki disease AND infliximab”. Bibliographies of selected references were also evaluated for relevant studies. Only articles published in English were included in this review.
Hui-Yuen et al. [3] demonstrated the critical role of tumor necrosis factor-alpha (TNF-α) in the development of CALs using an animal model of KD. During the acute phase of KD, elevated levels of various inflammatory cytokines, including TNF-α, are detected in the blood. TNF-α specifically plays a crucial role in inducing coronary artery inflammation and aneurysm formation in their animal model.
Hirono et al. [4] conducted a study comparing cytokine levels between KD patients and healthy controls, finding significantly elevated levels of cytokines, such as interleukin-6 and soluble TNF receptor-1, in KD patients. Following infliximab treatment, a marked reduction in these cytokine levels was observed, which correlated with decreased serum C-reactive protein (CRP) levels and resolution of fever.
Several studies have investigated the use of infliximab as part of the initial treatment regimen in combination with IVIG, comparing outcomes such as the incidence of CALs, fever duration, and hospital stay. While the addition of infliximab showed trends toward reducing IVIG resistance, the differences were not always statistically significant.
Tremoulet et al. [5] conducted a randomized, double-blind, placebo-controlled trial involving 196 patients to evaluate the efficacy of combining infliximab with IVIG as an intensification of initial treatment for KD. Infliximab-treated patients experienced fewer fever days and faster normalization of inflammatory markers, although there was no significant difference in the rates of IVIG resistance between the two groups. The infliximab group also showed a significant reduction in left anterior descending coronary artery Z score, but no difference in the overall incidence of CALs was observed between the treatment arms. Similarly, Han et al. [6] conducted a study in which 154 KD patients were randomly assigned to two groups: 77 received combined IVIG and infliximab therapy, while the other 77 received IVIG alone. The combined treatment group exhibited significantly fewer cases of refractory KD (5% vs. 18%, P < 0.001). Additionally, the combined treatment group demonstrated better outcomes, including shorter fever duration, reduced hospital stays, and a lower incidence of CALs. However, there were no statistically significant differences in the incidence of CALs between the two groups. Jones et al. [7] explored the efficacy of adding infliximab to the initial IVIG regimen in KD patients with pre-existing CALs. They found that patients receiving both IVIG and infliximab were significantly less likely to require additional therapy compared to those treated with IVIG alone (44% vs. 11%, p = 0.003). While no significant differences were observed in hospital stay, CAL size, or CRP reduction, there was a trend towards shorter hospital stays in the infliximab group.
Dionne et al. [8] conducted a retrospective analysis of high-risk North American KD patients with baseline CALs. These patients received either IVIG alone or combined therapy with infliximab (5 or 10 mg/kg) or corticosteroids. Intensification of initial treatment with either corticosteroids or infliximab was associated with a lower rate of subsequent coronary artery enlargement compared to IVIG alone. A meta-analysis by Li et al. [9] concluded that combining infliximab with IVIG as a primary treatment for KD reduces the rate of IVIG resistance compared to IVIG alone. While infliximab has demonstrated efficacy in improving treatment outcomes for refractory KD, this combination therapy did not confer a significant advantage in reducing the incidence of CALs.
According to Miyata et al. [10], combining infliximab with IVIG may offer superior efficacy over IVIG alone in treating high-risk KD patients presenting with initial coronary aneurysms (Z score ≥ 2.5). The study reported a higher rate of coronary aneurysm regression in patients treated with high-dose infliximab (10 mg/kg) compared to those receiving IVIG alone or low-dose infliximab (5 mg/kg). However, the study acknowledges limitations in stratifying high-risk patients and emphasizes the need for further research to validate these findings and resolve ongoing controversies regarding the long-term impact of initial infliximab therapy on coronary outcomes.
Accurately predicting IVIG resistance remains a significant challenge despite its potential to improve treatment outcomes for high-risk patients with CALs. While scoring systems are used in Japan, studies have demonstrated limited applicability in other populations. A meta-analysis by Kuniyoshi et al. [11], which included 161 model analyses from 48 studies, evaluated predictive models for initial IVIG resistance in KD. The summary C-statistics, including external validation results, were consistently below 0.75. The meta-analysis concluded that none of the existing scoring models (Kobayashi, Egami, Sano, and Harada) could reliably differentiate between patients who would develop IVIG resistance and those who would not. Lam et al. [12] have highlighted the limitations on relying on clinical data alone. Future predictive tools may require the integration of data from transcriptomics, proteomics, and genetics in addition to traditional clinical measures.
For refractory KD patients, options include a second IVIG infusion, intravenous meth ylprednisolone pulse (IVMP), or infliximab. Pan et al. [13] reported no significant differences in the risk of CAL development between second IVIG infusions, IVMP and infliximab. Although a second IVIG infusion (2 g/kg) is a common approach, there is a concern that repeated IVIG doses may increase the risk of hemolytic anemia [14], and this strategy has not been adequately tested in a randomized trial with sufficient statistical power.
For refractory KD, IVMP at a dose of 30 mg/kg over 2–3 hours daily for three consecutive days offers a lower-cost alternative to infliximab (5–10 mg/kg single dose). However, IVMP is associated with higher risks of side effects, such as bradycardia, hypertension, hyperglycemia, and hypothermia. Although IVMP may lead to faster fever resolution initially, some patients experience “masked fever” that recurs later, potentially delaying treatment. The optimal dosing regimen for IVMP — whether a single dose, three daily doses, or an additional oral steroid taper — remains unclear in confirming treatment response. A Cochrane meta-analysis [15] suggested that early corticosteroid therapy in KD might improve coronary artery outcomes, reduce hospitalization duration, and shorten symptom duration. However, the quality of evidence supporting these benefits is moderate. Ikbata et al. [16] found that patients with refractory KD receiving corticosteroid therapy might have a higher risk of CALs than those not receiving corticosteroids.
Infliximab, a TNF-α inhibitor, has been approved for treating immune-mediated inflammatory disorders such as Crohn's disease and juvenile idiopathic arthritis. Several studies have demonstrated its efficacy as an alternative treatment for refractory KD. Burns et al. [17] conducted a multicenter, randomized, prospective trial to assess the safety, tolerability, and pharmacokinetics of infliximab in children with IVIG-resistant KD. Twenty-four patients received either a second IVIG infusion (2 g/kg) or infliximab (5 mg/kg). Infliximab was associated with fever resolution within 24 hours in 11 out of 12 patients (92%), compared to 8 out of 12 (67%) in the IVIG group. There were no significant differences between the groups in changes from baseline in laboratory variables, fever resolution, or echocardiographic assessment of coronary arteries.
A retrospective multicenter study by Song et al. [18] found similar results, with 80% of refractory KD patients responding within 12 hours of infliximab infusion, showing complete resolution of clinical signs and symptoms. Additionally, CRP levels decreased in most patients after infliximab administration. A retrospective study by Son et al. [19] compared infliximab with IVIG as a retreatment option for refractory KD and reported faster fever resolution and shorter hospitalization in the infliximab group, without significant differences in coronary artery outcomes or adverse events. Youn et al. [20] conducted a study involving 43 patients with KD resistant to initial IVIG treatment, with patients randomly assigned to receive either a second IVIG (n = 32) or infliximab (n = 11). The infliximab group had a higher response rate (91%) compared to the IVIG group (66%). Patients treated with infliximab experienced shorter fever duration and hospitalization, although there were no significant differences in coronary artery outcomes or adverse events between the two groups.
A meta-analysis by Xue et al. [21] found that TNF inhibitors were not associated with a significantly lower risk of treatment resistance compared to IVIG in KD. While TNF inhibitors reduced fever duration, they did not exhibit a cardioprotective effect in refractory KD patients. The risk of serious adverse events was comparable between the two groups. A Korean retrospective multicenter study by Hur et al. [22] investigated the efficacy of infliximab for refractory KD and its impact on CALs. Among 102 patients divided by infliximab timing, the study found significantly shorter fever duration and a lower incidence of significant coronary aneurysms in the early treatment group. These findings suggest potential benefits of early infliximab administration in reducing the risk of CALs, but larger, prospective randomized studies are required to confirm these results.
In Japan, a nationwide survey and a study by Sonoda et al. [23] investigated the efficacy of infliximab in treating 76 patients with refractory KD. While 10 patients (15%) developed CALs and 3 had coronary aneurysms within a month of disease onset, all cases showed regression or suppression of CALs by the end of follow-up. These findings suggest that infliximab may accelerate the regression of CALs in refractory KD. A nationwide Japanese survey by Masuda et al. [24] analyzed data from 434 KD patients treated with infliximab, primarily as a third-line therapy (63%). The study could not directly assess coronary artery changes before and after treatment but reported a 7% incidence of newly developed CALs in refractory KD patients without pre-existing CALs, and a 16% worsening of CALs in those with pre-existing lesions following infliximab treatment. Although this retrospective study without a control group cannot definitively establish infliximab’s efficacy, the findings suggest that earlier infliximab treatment may be effective in reducing CALs development.
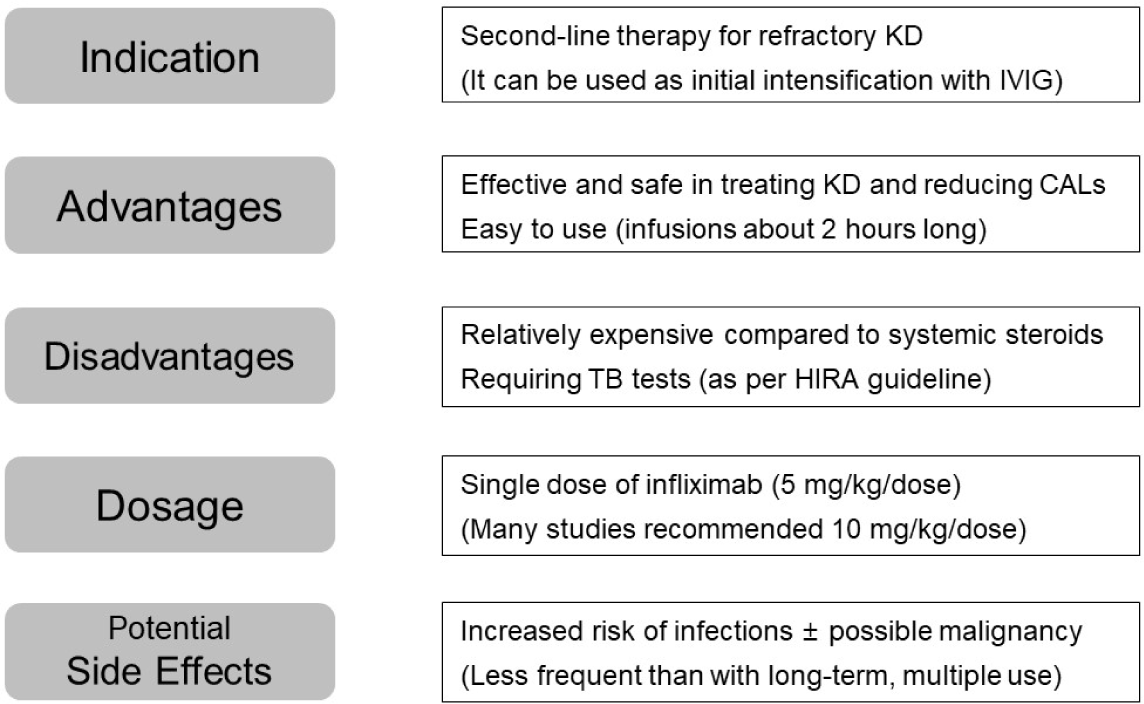
Infliximab treatment has been shown to be more effective than a second IVIG infusion in reducing inflammation, fever, and hospitalization duration in KD patients. One key advantage of infliximab is its faster administration time compared to IVIG. While IVIG can take up to 12 hours to administer, infliximab is typically administered within 2 hours [1]. Early recognition of treatment response allows for faster treatment adjustments if needed to mitigate the progression of CALs. These findings challenge traditional recommendations by the AHA and Japanese guidelines, which advocate for a second IVIG infusion as the preferred treatment for IVIG-resistant KD [1,25,26].
The AHA guidelines recommend a standard infliximab dose of 5 mg/kg, administered intravenously over 2-hour period. However, Vande Casteele et al. [27] found that administering infliximab after IVIG can result in a 50% reduction in the volume of distribution in the peripheral compartment. This pharmacokinetic interaction likely contributed to the decision to increase the standard infliximab dose to 10 mg/kg in the United States. While the 5 mg/kg dose has been associated with a low incidence of serious adverse events, further research is needed to evaluate the efficacy and safety of the higher 10 mg/kg dosage in KD patients. A multivariable logistic regression analysis by Miyata et al. [10], demonstrated a significant association between IVIG plus 10 mg/kg infliximab and a higher likelihood of CALs regression compared to IVIG alone. These findings suggest that high-dose infliximab may be particularly beneficial for patients presenting with CALs at diagnosis. Although the Korean Health Insurance Review & Assessment Service (HIRA) has covered infliximab for KD patients since 2021, HIRA’s policies currently limit its use to a single dose of 5 mg/kg and prohibit co-administration with IVIG.
While infliximab is generally well-tolerated in KD patients, infusion reactions were reported in 1.4% of patients (4 out of 294) in the SAKURA Study [19,28]. Although concerns have been raised regarding a potential link between infliximab and an increased risk of infections and malignancy, current research has not established a definitive association with malignancy [29,30]. Unlike other diseases such as rheumatic disease or inflammatory bowel disease, KD treatment typically involves a single infliximab infusion, which may contribute to the lower incidence of adverse events observed in KD patients.
Infliximab use under HIRA’s policies for KD patients is subject to latent tuberculosis screening protocols. This necessitates a tuberculin skin test or interferon-gamma release assay prior to treatment, which may delay the timely administration of infliximab in high-risk refractory KD patients due to the waiting period for test results. Although concerns exist about the reactivation of latent tuberculosis with infliximab use, no such cases have been reported in KD patients receiving a single dose of infliximab as adjunctive therapy.
There have been reports concerning the safety of infliximab therapy in KD patients who have received live vaccines [31]. According to Japanese guidelines for the use of infliximab in KD, it is recommended to administer infliximab at least three months after receiving live vaccinations, or six months after the BCG vaccine [28]. However, recent studies have not demonstrated an increased risk of vaccine-related infections [28,31]. Currently, data on the optimal interval between live vaccination and infliximab administration are limited and warrant further study.
Conclusion
When used as part of initial treatment in combination with IVIG, infliximab has been associated with shorter fever durations and reduced hospital stays, and may potentially lower the rate of IVIG resistance. Although there is no conclusive evidence that infliximab reduces the incidence of CALs, recent studies suggest that high-dose (10 mg/kg) infliximab as an adjunct to initial therapy may be beneficial for patients presenting with coronary aneurysms at diagnosis, potentially improving CALs regression. Infliximab, when used as a retreatment option for refractory KD, has shown reductions in inflammation, fever, and hospitalization duration. While some studies have indicated that infliximab might reduce the incidence and progression of CALs, the majority of reports have not found significant differences between infliximab and IVIG in this regard. Fig. 1 highlights the key aspect of infliximab use in the treatment of KD.