Introduction
Multisystem inflammatory syndrome in children (MIS-C) represents a profound medical challenge, defined by its hallmark acute systemic inflammation and multi-organ dysfunction, which exhibits notable parallels to Kawasaki disease (KD), Kawasaki disease shock syndrome (KDSS), and toxic shock syndrome (TSS). The global coronavirus disease of 2019 (COVID-19) pandemic, declared in 2019, has coincided with a marked surge in the prevalence of these pediatric manifestations, notably throughout Europe and the United States, commencing predominantly from April 2020 [1-4]. Despite the similarities with Kawasaki disease, MIS-C has been recognized as a distinct medical condition, which is characterized by a pronounced hyperinflammatory shock and multi-organ dysfunction. The temporal correlation with the pandemic and the amassed case evidence has underscored a compelling epidemiological association with COVID-19, pinpointing the aberrant immune response following severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) infection as a pivotal etiological factor of MIS-C [5,6].
Contrary to the typical mild symptomatology observed in primary SARS-CoV-2 infections in children [7], MIS-C has evolved as a matter of considerable international concern due to its potential to deteriorate into a life-threatening illness. Particularly, the cardiovascular complications, encompassing ventricular dysfunction and coronary aneurysm associated with MIS-C, necessitate urgent attention. These ramifications have not only amplified recognition of this disease but have also situated MIS-C in conjunction with KD as a significant acquired heart disease in pediatric and adolescent populations.
In view of the critical nature of MIS-C and its significant cardiac implications, an exhaustive exploration of the cardiac sequelae of this syndrome is pivotal. Hence, this review article is directed toward a comprehensive examination of existing research focusing on the cardiac outcomes in children with MIS-C. By delineating the current understanding of the cardiovascular complications and exploring their respective treatment strategies, this review aims to contribute to the advancements in the clinical management and prognostic outcomes of pediatric patients diagnosed with MIS-C.
Main Subject
MIS-C is a rare complication that can occur three to six weeks following SARS-CoV-2 infection. This syndrome is characterized by its acute systemic inflammation and ensuing multi-organ dysfunction [8]. Case definition criteria for MIS-C, as outlined by the Council of State and Territorial Epidemiologists (CSTE) / Centers for Disease Control and Prevention (CDC) in 2023, include: (1) patient age ≤ 21 years, (2) a minimum fever of 38℃, (3) clinical evidence necessitating hospitalization due to severe illness, (4) laboratory proof of systemic inflammation (CRP ≥ 3 mg/dL), (5) two or more organ system involvement, (6) an antecedent SARS-CoV-2 infection, and (7) an absence of any alternative diagnoses [5] (Table 1). Contrastingly, the World Health Organization’s (WHO) case definition covers children and adolescents aged 0–19 presenting with a fever persisting over three days but without the necessity for hospital admission due to severe illness [6]. The Korea Disease Control and Prevention Agency (KDCA) similarly focuses on the 0–19 age group and broadens diagnostic criteria to include cases in which a fever of 38 degrees or higher persists for over 24 hours [9]. It is important to acknowledge that the range of illnesses included within the case definition is extensive, and MIS-C represents a spectrum of clinical patterns and varying degrees of severity rather than a specific, singular clinical feature (Table 1).
indicated by left ventricular ejection fraction < 55 %, coronary artery dilatation, aneurysm, or ectasia, or troponin elevated above laboratory normal range.
indicated by rash, inflammation of the oral mucosa, conjunctival injection, conjunctivitis, or erythema of the hands or feet.
MIS-C: Multisystem Inflammatory Syndrome in Children; CSTE/CDC: Council of State and Territorial Epidemiologists/Lefts for Disease Control and Prevention; WHO: World Health Organization; KDCA: Korea Disease Control and Prevention Agency; CRP: C-reactive Protein; ESR: erythrocyte sedimentation rate; LDH: lactate dehydrogenase; IL-6: interleukin-6; SARS-CoV-2: severe acute respiratory syndrome coronavirus 2; RNA: ribonucleic acid; COVID-19: coronavirus disease-2019; RT-PCR: reverse transcriptase polymerase chain reaction.
Efforts have been made to enhance our understanding of MIS-C by classifying it based on clinical characteristics. The United Kingdom’s (UK) Paediatric Inflammatory Multisystem Syndrome Temporally associated with SARS-CoV-2 (PIMS-TS) National Consensus classified MIS-C into two types: KD-like and nonspecific type [10]. Concurrently, the CDC’s Morbidity and Mortality Weekly Report enumerated three categories: MIS-C without concomitant acute COVID-19 or KD, MIS-C with KD overlap, and MIS-C with severe acute COVID-19 infection overlap [11]. Despite these classification variations, they generally encompass a broader framework that differentiates between a KD-like type and a hyperinflammatory shock type (either nonspecific type or MIS-C without acute COVID-19 or KD co-occurrence). The KD-like type mirrors the clinical pattern of complete and incomplete KD and typically demonstrates less severe cardiovascular involvement and lower hemodynamic instability relative to the nonspecific type. This type often correlates with a higher incidence of coronary aneurysms and mucocutaneous symptoms and primarily impacts younger children [10-12]. Conversely, the nonspecific type, or MIS-C without KD or acute COVID-19 overlap, is characterized by hyperinflammation, hemodynamic instability, and the involvement of multiple organ systems. Nearly all patients in this group experience gastrointestinal involution, along with cardiac dysfunction and shock [10-12]. As case definitions evolve, the existing classification may be revised, especially with KD now regarded as a potential alternative diagnosis [13]. The specific pattern of cardiac complications observed in each clinical type could direct variations in therapeutic strategies.
The precise incidence of MIS-C remains elusive. Initial studies conducted in the United States during the pandemic have underscored its relative infrequency. Estimates place the incidence at approximately 2 per 100,000 individuals, constituting less than 1% of SARS-CoV-2 infections in individuals aged 21 or younger. For context, the overall incidence of SARS-CoV-2 infection within the same demographic stood at 322 per 100,000 [14]. Recent epidemiological surveys have documented a marked decrease in the incidence of MIS-C. This downward trend likely correlates with the shift in prevalent SARS-CoV-2 variants during the course of the pandemic. While exact figures may exhibit some discrepancies across different reports, an undeniable downward trend in MIS-C cases has been observed. For instance, during the pre-Delta/ancestral phase, incidence fluctuated between an estimated 25 to 130 cases per 100,000 SARS-CoV-2 infections. During the Delta phase, incidence further declined to around 50 cases per 100,000, and during the Omicron phase, it contracted to a mere 4 to 8 cases per 100,000 [15-18].
As the SARS-CoV-2 variants of concern change, a notable reduction in the severity of MIS-C symptomatology has been recorded. This decrease has led to enhanced cardiac outcomes, typified by shortened hospital stays, a reduction in ICU admissions, and decreased reliance on interventions such as vasopressors and mechanical ventilation [15]. The widespread implementation of mRNA-based vaccination against COVID-19 has significantly contributed to the reduction of MIS-C incidence. Holm et al.’s comparative analysis of MIS-C incidence in vaccinated children experiencing breakthrough SARS-CoV-2 infections versus their unvaccinated counterparts revealed respective incidence rates of 290.7 and 101.5 cases per 1,000,000 population during the Delta phase, and 34.9 and 3.7 cases per 1,000,000 population during the Omicron phase [18]. A US-based study assessing 304 MIS-C cases against 502 controls reported a significant correlation between MIS-C and reduced vaccination rates, with an adjusted odds ratio of 0.16 [19]. In summary, over the course of the pandemic, the incidence of MIS-C has seen a gradual decrease. This trend likely reflects a convergence of factors such as changes in the dominant SARS-CoV-2 variants, extensive uptake of vaccination, and advancements in therapeutic interventions.
MIS-C predominantly affects children aged between 6 and 12 years, with a marginally greater incidence observed in males relative to females at a ratio of 6:4 [4,11,14,20-22]. A possible association has been suggested between the frequency and severity of MIS-C and obesity, although further investigation is necessary to confirm this relationship [23-25]. With regard to ethnicity, a heightened prevalence of MIS-C has been detected among non-Hispanic black and Hispanic populations [22,26,27]. This trend is congruent with the elevated incidence of SARS-CoV-2 infection within these demographic groups. Yet, even when taking into account the proportional number of MIS-C cases in relation to the total SARS-CoV-2 infections, the incidence remains disproportionately high among non-Hispanic black and Hispanic individuals [26,27]. The precise causes of these demographic patterns are not yet fully understood. Socioeconomic factors might indeed play a role in this inequality, yet research has also delved into the potential effects of variations in angiotensin-converting enzyme 2 (ACE-2) gene expression, a factor that influences viral tropism in SARS-CoV-2, along with differences in immune responses attributed to age and sex-specific factors [28-30].
According to the KDCA, between May 2020 and February 2022, 19 MIS-C cases have been documented in individuals younger than 18 years out of a cumulative 1,190,860 SARS-CoV-2 infections in Korea [31]. This equates to an incidence rate of 1.6 MIS-C cases per 100,000 SARS-CoV-2 infections, a significantly lower rate than those reported in other nations. Given the prevalence of the Omicron variant in Korea starting from December 2021, this incidence rate of MIS-C solely captures the pre-Omicron period, while the actual occurrence is anticipated to be significantly greater. Nevertheless, the notably decreased incidence of MIS-C in Korea may be attributable to the nation’s lower infection rate prior to the Delta variant surge and before extensive vaccination deployment [32]. Yet, the potential influence of ethnic and socioeconomic elements cannot be overlooked.
Although MIS-C can result in severe illness, the majority of affected patients exhibit satisfactory recovery following appropriate treatment, and the mortality rate is infrequent [33-35]. As per the data published by the CDC in June 2023, 9,480 cases of MIS-C have been reported in the United States, with approximately 79 cases leading to fatalities, yielding a mortality rate of about 0.8% [36]. Nonetheless, a separate cohort study concentrating on critically ill patients revealed a higher mortality rate, reaching up to 5% [37].
MIS-C, although distinct from KD, presents a diagnostic challenge in clinical settings due to significant symptomatic overlap [38,39]. Previous case series have reported that a notable proportion of MIS-C patients, ranging from 5.9% to 11%, meet the diagnostic criteria for complete KD [11,12], while a substantial percentage (40% to 50%) meet the criteria for Incomplete KD [2,3,14,20,40]. The similarity in clinical manifestations is further compounded by overlapping pathophysiological features. In terms of immunopathogenesis, the interleukin-1ß pathway is upregulated in both KD and MIS-C. A study, guided by artificial intelligence (AI), demonstrated that KD and MIS-C share a continuum of host immune response, albeit with nuanced variations in the detailed immunopathogenesis [41]. In response to these diagnostic complexities, the CDC devised a scoring system in 2022 to facilitate the differentiation of MIS-C from COVID-19, KD, and TSS [42].
When examining the epidemiological characteristics of MIS-C, it is pertinent to mention that KD is predominantly prevalent in East Asian populations, while MIS-C primarily affects non-Hispanic black individuals [22,26,27,43,44]. Furthermore, the age distribution of MIS-C typically ranges from 6 to 12 years, diverging from KD, which predominantly affects children under 5 years of age [4,11,14,20-22,43,44]. In terms of symptomatology, MIS-C is often characterized by gastrointestinal involvement, shock, and ventricular dysfunction. In contrast, mucocutaneous involvement and the development of coronary aneurysms are more commonly associated with KD [42,44,45]. Moreover, MIS-C can be differentiated by lower nadir platelet and lymphocyte counts, alongside higher peak C-reactive protein (CRP) levels [42,44]. The vigilant observation of these distinct features in clinical settings and their integration into the CDC’s scoring system can potentially streamline accurate differential diagnosis.
Cardiovascular manifestations are frequently associated with MIS-C, ranking third behind fever and gastrointestinal symptoms. Notably, cardiovascular involvement is observed in more than 75% of MIS-C patients [35,38], a contrast to the acute phase of KD, which exhibits a relatively low cardiac involvement that is present in only 9% of cases [43,46]. Clinical consequences span a broad spectrum of cardiovascular complications, ranging from subclinical presentations to more severe conditions, such as ventricular dysfunction, pericarditis, coronary aneurysms, conduction abnormalities, and even shock and cardiac death (Table 2).
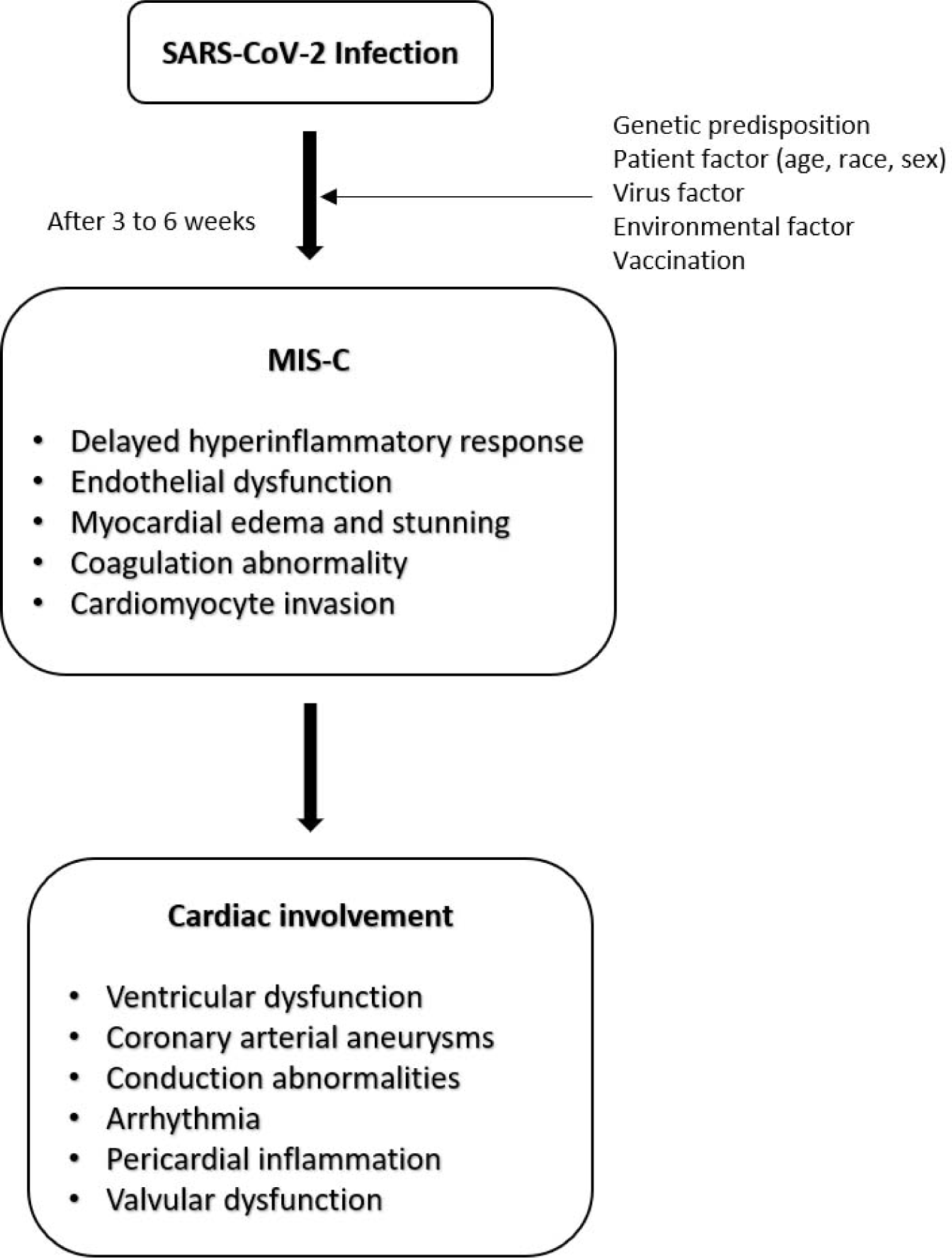
Despite its high prevalence, the precise pathophysiological mechanisms underlying cardiovascular involvement in MIS-C remain poorly understood, resulting in various contrasting hypotheses [47]. A prominent body of research postulates MIS-C as a post-viral inflammatory response manifesting 3 to 6 weeks post SARS-CoV-2 infection, particularly in genetically predisposed children [39,48-51]. As such, it is plausible that the inflammatory processes and cytokine storm following infection significantly contribute to cardiovascular involvement. The hyperinflammatory response in MIS-C is often accompanied by endothelial dysfunction and microangiopathy, vascular inflammation, myocardial edema, and stunning. These factors constitute the principal mechanisms contributing to the development of cardiovascular complications (Fig. 1).
Alternatively, there exists an ongoing debate regarding the possibility of direct myocardial damage caused by the infiltration of SARS-CoV-2 into cardiac tissues [38]. Notably, SARS-CoV-2 exhibits affinity toward cardiovascular cells expressing the ACE-2 receptor, thereby indicating its tropism for cardiovascular tissue. Furthermore, some studies posit MIS-C as a persistent infection, characterized by an unceasing hyperinflammatory response induced by SARS-CoV-2 that has not been fully eradicated [49,52]. The elucidation of these complex pathophysiological mechanisms, made possible through continuous research, is vital for a comprehensive understanding of MIS-C and its cardiovascular implications.
The primary and most prevalent cardiac manifestation in MIS-C is myocardial dysfunction [53]. Myocardial injury is confirmed through elevated serum levels of troponin and brain natriuretic peptide (BNP) [54], while echocardiograms demonstrate a spectrum of abnormalities that can range from minor strain irregularities to severe systolic dysfunction [48]. Among these, left ventricular (LV) systolic dysfunction is most closely associated with hemodynamic instability in patients [55]. Initial reports from the UK and Italy indicated a depressed LV ejection fraction (EF) in 75% and 50% of patients respectively [1,2], while subsequent broader cohort studies reported a prevalence of approximately 34% [34,35]. Nevertheless, meta-analytical data suggest that LV systolic dysfunction occurs in nearly half of all patients [51]. Mild systolic dysfunction (EF 45% to 55%) is the most common, observed in 55% of all patients, whereas severe dysfunction (EF 35% or less) is less common, accounting for 22% of patients [34]. Severe hemodynamic instability necessitating extracorporeal membrane oxygenation (ECMO) is identified in 3% of all patients [34,53]. LV systolic dysfunction bears significant clinical implications due to its strong association with the use of inotropic agents and intensive care unit (ICU) management [35,53].
In instances of subclinical myocarditis, where significant systolic dysfunction is absent, myocardial strain abnormalities have demonstrated a higher diagnostic sensitivity for myocardial injury [56]. A body of research suggests that in MIS-C, myocardial strain, coupled with an increase in cardiac biomarkers, offers superior diagnostic sensitivity for myocardial injury compared to a reduction in ejection fraction alone [57-60]. Additionally, diastolic dysfunction is not only more intricately linked with myocardial injury than systolic dysfunction, but it also tends to persist over an extended period, even beyond the recovery of systolic function [57].
Alongside the abnormalities found in functional studies, pericardial effusion is a common finding on echocardiograms in patients with MIS-C. It has been reported that pericardial effusion is present in 19% to 66% of MIS-C patients [33-35,51,57,61]. Most patients experience successful recovery within a few months, marked by the resolution of clinical manifestations and ventricular dysfunction. However, rare instances of fatal pericardial tamponade have been reported in MIS-C patients [62,63]. Valvular regurgitation has been reported in 42% to 65% of MIS-C patients, with a predominance of involvement in the mitral valve [33,35,61,64].
Cardiac magnetic resonance imaging (MRI) studies conducted in the acute phase of MIS-C in patients exhibiting cardiac dysfunction have revealed that the majority of these patients fail to satisfy the Lake Louise criteria for the diagnosis of acute myocarditis [61,65,66]. The late gadolinium enhancement (LGE), indicative of focal myocardial necrosis and fibrosis, was only observed in a small subset of patients [61,65,66]. These observations suggest that myocardial edema or stunning, resulting from the inflammatory process, may be the principal causative mechanisms underlying cardiac dysfunction in MIS-C, rather than myocardial destruction and necrosis [47]. This hypothesis is further substantiated by the rapid recovery of cardiac function and the significant increase in BNP among cardiac biomarkers [34,53].
In contrast, postmortem analyses of five pediatric patients succumbing to COVID-19 and MIS-C confirmed the expression of the SARS-CoV-2 antigen within the cardiac endothelium and myocardium. This was accompanied by myocardial necrosis and perivascular inflammatory infiltration in three instances of MIS-C-related deaths [67]. Such findings implicate direct viral invasion and cellular destruction as potential etiological factors for myocardial dysfunction in fatal scenarios. It is, however, important to underscore that these conclusions have been drawn from a limited number of case studies, thereby complicating the extrapolation of direct viral inflammation as a generalized pathophysiology in MIS-C.
The outlook for ventricular dysfunction in MIS-C patients appears largely positive. The majority experience a rapid amelioration of systolic dysfunction within one to two weeks, signifying ventricular function restoration upon discharge [34,35,53,57]. Notably, about 99% of these patients regain normal ventricular function within a three-month span [34]. The initial dysfunction severity, as observed on echocardiography, does not appear to influence the likelihood of ejection fraction recovery [34]. Moreover, even in instances where cardiac MRI reveals myocardial necrosis, there is no discernible difference in the rate of LV function recovery in comparison with patients without LGE [66]. This suggests that the occurrence of LGE may not necessarily be indicative of persistent LV dysfunction. However, a longitudinal study investigating MIS-C patients’ outcomes up to one year reported LGE findings in 50% of patients who exhibited cardiovascular involution after nine months [33]. This could imply that the imaging findings might persist over an extended duration. Given the limited understanding of the long-term prognosis in patients with myocardial necrosis findings on cardiac MRI, regular cardiological follow-up studies are warranted to further elucidate the clinical implications.
MIS-C and KD share striking similarities, with both displaying a predilection for coronary arterial abnormalities. Among untreated KD patients, roughly 40% demonstrate coronary artery involvement, with persistent coronary artery aneurysms observed in 15%–25% of these cases [68]. Notably, a subset of these patients (4%–6%) develop giant coronary arterial aneurysms that persist despite treatment [69]. However, MIS-C presents with coronary artery involvement in a range of 24%–48% of cases [2,4,14,20,49,57,70,71]. This is further corroborated by meta-analyses, which estimate the prevalence to be approximately one in five patients [38,51]. Within the spectrum of coronary artery aneurysms, small aneurysms with a z-score ≥ 2.5 are reported in 4%–15% of cases, while moderate aneurysms with a z-score ≥ 5 occur in approximately 3%–7% of cases [20,35,57,70]; giant aneurysms are relatively uncommon [34,35]. Short-term follow-up studies demonstrate a favorable course, with most coronary aneurysms in MIS-C showing improvement, suggesting comparatively milder coronary involvement compared to Kawasaki disease [57,71]. Consequently, it seems that MIS-C might display a relative sparing of the coronary arteries in comparison to KD.
Patient stratification of MIS-C based on clinical features highlights a subset of individuals who present a KD-like phenotype. This is predominantly observed in younger patients below the average age, often associated with coronary arterial dilation [11,12]. Interestingly, a study focusing on MIS-C patients under the age of 5—a demographic below the average age of MIS-C cases—reveals the presence of coronary aneurysms (with a z-score > 2.5) in approximately 30% of patients and coronary ectasia (with a z-score ranging from 2 to 2.5) in 40% of patients [72]. Moreover, true coronary aneurysms have been infrequently documented in the Multisystem Inflammatory Syndrome - Adult (MIS-A) case series involving young adults aged 21 and above [45]. McMurray et al. have proposed that the higher incidence of giant aneurysms in young infants, compared to older MIS-C patients, may stem from mechanical stress induced by edema of the internal elastic lamina, which consequently impacts the microanatomy of blood vessels during the growth process [38].
Several studies have underscored coronary artery abnormalities, such as peri-coronary echogenicity or prominent coronary arteries [1,73,74]. However, such observations cannot be quantified or standardized, imposing limitations on their interpretive value [48,57]. Although a prominent coronary artery is a frequent finding in KD, it may also result from coronary artery dilatation due to the myocardium’s elevated oxygen demand in conditions such as viral myocarditis and febrile illnesses [75,76]. Therefore, a prudent interpretation of these observations is warranted. Notably, peri-coronary echogenicity was mentioned in the 2004 American Heart Association (AHA) guidelines for Kawasaki disease but was omitted from the 2017 AHA guidelines due to its non-quantifiable nature. Consequently, Matsubara et al. postulated that utilizing peri-coronary echogenicity as a diagnostic marker for MIS-C could potentially inflate the estimation of coronary arterial aneurysms [57].
The precise etiology of coronary artery aneurysm formation in MIS-C remains largely elusive. The primary causes of coronary arterial dilatation and edema in MIS-C are deemed to be circulating cytokines and endothelial cell dysfunction [38,50,57]. Conversely, in Kawasaki disease, the pathogenesis involves direct inflammatory cell infiltration around the coronary artery, accompanied by the disruption of structural integrity due to the direct compromise of collagen and elastin fibers, factors that may promote aneurysm development [50,77]. Encouragingly, the majority of coronary artery abnormalities in MIS-C exhibit spontaneous resolution within a span of 30 days [34]. Nevertheless, it is critical to emphasize that while coronary artery involvement in MIS-C tends to be less severe compared to Kawasaki disease, the development of giant coronary aneurysms still poses a significant risk, particularly in young patients [72]. Future investigations are warranted to enhance diagnostic methodologies and develop targeted therapies aimed at preventing the formation of coronary aneurysms.
Electrocardiogram (ECG) abnormalities have been shown to manifest in 35% to 67% of patients with MIS-C, often in the form of nonspecific changes accompanied by myocardial changes [35,78]. The most frequently observed findings in patients with MIS-C included low QRS amplitude and flattened T waves in the precordial leads suggestive of underlying myopericarditis [78]. Approximately 40%–50% of patients exhibited T wave inversion [78-80]. Additionally, QTc prolongation, an indicator of myocardial inflammation and edema, was present in 22%–67% of the studied cases [78-80]. These repolarization abnormalities, along with conduction delays reflected in prolonged PR, QRS, and QTc intervals, signify the impact of inflammatory changes within the myocardium and conduction tissue [78]. Furthermore, significant ST-segment changes were reported in 7.9% of MIS-C cases, primarily transient and rarely indicative of a pericarditis pattern or coronary ischemia [78]. Although these changes were mostly transient, they rarely indicated a pericarditis pattern or coronary ischemia.[78] Notably, the majority of these ECG abnormalities exhibited a pattern of rapid resolution upon discharge [78,79,81]. Among the observed bradyarrhythmias, first-degree AV block was the most common, affecting 6.3% to 25.4% of cases [35,78,81-83]. Typically, these cases manifested around six days following the onset of fever, resolved within a week of symptom onset, and did not necessitate specific therapeutic interventions [81-83]. In 37% of patients, an abnormal PR: HR slope, a paradoxical prolongation of the PR interval in response to an increased heart rate, was observed [78]. Despite associations with poor prognoses in adult COVID-19 infections [84], abnormal PR: HR slopes generally portend a favorable outcome in MIS-C patients [78,83]. Nevertheless, it should be noted that first-degree AV block has the potential to escalate into higher-degree blocks, occasionally necessitating the insertion of a permanent pacemaker [82,85]. Therefore, continuous telemetry is recommended in cases where AV block is evident on the ECG [82]. Continuous junctional rhythm, another rare bradyarrhythmia, has been reported in 2% to 3% of MIS-C cases [78,79].
Despite its infrequency, tachyarrhythmia represents a significant complication indicative of severe cardiac involvement and an unfavorable prognosis [86]. In a study by Dionne et al., tachyarrhythmias were identified in 41 (1.7%) out of 2,343 MIS-C patients. Of these, ventricular tachycardia (VT) accounted for 66%, supraventricular tachycardia (SVT) 39%, and accelerated junctional rhythm 20%. Notably, VT was specifically associated with high mortality, especially in patients with acute severe COVID-19 infection. Among the 41 MIS-C patients with VT, there were two fatalities, a lower mortality rate compared to the 36.3% observed among patients with acute severe COVID-19 infection [86]. Tachyarrhythmia in the context of MIS-C correlates with increased illness severity, heightened incidence of concomitant cardiac complications, prolonged hospitalization, and elevated in-hospital mortality rates [86]. It is important to note that while rare, severe conduction abnormalities such as tachyarrhythmia or high-degree AV block necessitate judicious management in MIS-C patients.
Patients suspected of MIS-C who are well-appearing with stable vital signs and a reassuring physical examination may be adequately managed through close outpatient clinic follow-up [10,87]. Nevertheless, a considerable proportion of MIS-C cases present with moderate to severe illness, thereby requiring hospitalization [48,53]. Comprehensive management of these hospitalized patients necessitates an integrative approach that combines supportive care with immunomodulatory interventions [10,48,87]. The presence of concurrent cardiac complications often results in hemodynamic instability among MIS-C patients, thereby warranting the involvement of a multidisciplinary team of intensivists, infectious disease specialists, hematologists, neurologists, and pediatric rheumatologists, along with primary care provision by pediatric cardiologists [10,53,87].
In children exhibiting signs of cardiac involvement (elevated cardiac enzymes, abnormal coronary arteries, or cardiac dysfunction detectable on echocardiogram), specialized care within an ICU setting should be provided [53]. The primary objective in these patients is to promptly stabilize their vital signs [88]. In cases of poor cardiac output and arrhythmia, interventions such as inotropic support and mechanical ventilation are warranted [48,49,51,87]. If patients do not respond adequately even with high doses of inotropic support and medical treatment, ECMO is needed to maintain cardiac output and tissue oxygenation. Diagnostic markers such as elevated BNP and/or troponin levels at the time of diagnosis are associated with cardiac involvement and should be monitored until normalization [53,70,87]. Regular ECG follow-ups every 48 hours are essential [87]. Continuous telemetry is recommended to identify potential conduction abnormalities [80,82]. Initial follow-up echocardiography should be conducted within a one-to-two-week period, with subsequent examinations between four to six weeks if the patient exhibits favorable clinical progress [68]. More frequent examinations are necessary if there is evidence of LV dysfunction or coronary arterial aneurysm [10,68,87]. In instances where significant LV dysfunction was observed during the acute phase of the illness, a cardiac MRI is recommended between two to six months post-diagnosis to assess myocardial characterization and detect fibrosis [59,65,87]. Since the long-term prognosis for cardiac complications of MIS-C extending over at least one year remains unclear, further research is needed for systematic follow-up management.
Immunomodulatory treatment strategies are paramount in the management of MIS-C, aiming to stabilize patients with life-threatening manifestations such as shock while mitigating the risk of long-term complications, including coronary aneurysms [10,87]. Typically, a hierarchically structured approach is adopted for immunomodulatory treatment, with intravenous immunoglobulin (IVIG) and low-to-moderate-dose glucocorticoids serving as first-line interventions in the majority of instances (Table 3) [87,89]. When severe cardiac dysfunction hinders intravascular volume loading, a two-day administration of IVIG can be considered [10,87,89].
Although randomized controlled clinical trials directly comparing initial immunomodulatory treatments for MIS-C are lacking, several cohort studies have compared the efficacy of combination therapy involving IVIG and glucocorticoids with IVIG monotherapy. For example, Belhadger et al. conducted a comparative study with an IVIG-only cohort (18 patients) and a combination therapy cohort (22 patients), registering a hastened recovery time of cardiac systolic and diastolic function in the latter group [90]. Similarly, Ouldali et al. reported that the combination therapy cohort (33 patients) showed a decreased likelihood of necessitating second-line therapy, hemodynamic support, and acute left ventricular dysfunction following initial therapy and also had a shortened intensive care unit stay compared to an IVIG-only cohort (72 patients) [91]. In a large multi-center study involving 518 patients with MIS-C, Son et al. found that combination therapy was associated with a decreased risk of cardiovascular dysfunction, shock requiring vasopressor use, and adjunctive therapy compared to IVIG alone [92]. In contrast, McArdle et al., in their international multi-center cohort study with 614 patients, discovered that while combination therapy was correlated with a diminished necessity for adjunctive immunomodulatory therapy, it did not exhibit a significant difference in recovery from MIS-C between the treatment groups [93].
In cases where there is an inadequate response to initial treatment with IVIG and low-to-moderate-dose glucocorticoids, high-dose IV pulse corticosteroids (10–30 mg/kg/day) should be considered, especially in patients requiring multiple inotropes (Table 3) [87,89]. Administration of a second dose of IVIG is not recommended in such instances due to the potential risks of volume overload and hemolytic anemia [87]. Several biological agents are used for refractory disease in those with contraindications for prolonged glucocorticoid use (Table 3). Anakinra, an IL-1 receptor antagonist, may serve as a steroid-sparing agent for patients presenting with macrophage activation syndrome features [87,89]. Infliximab, a monoclonal antibody targeting TNF-α, represents another alternative biological agent with a steroid-sparing effect. However, it should not be administered if patients exhibit features of macrophage activation syndrome [87,89]. In order to mitigate the risk of rebound inflammation in MIS-C patients, a tapered regimen of steroid treatment over a period of two to three weeks may be required under the guidance of systematic laboratory testing and cardiac evaluations [87].
It remains unclear whether patients with MIS-C are at a higher risk of thrombosis compared to other critically ill patients. However, abnormalities in the coagulation cascade, notable elevations in D-dimer and fibrinogen levels, and increased clot strength have been reported, raising concerns about the potential thrombotic risk in MIS-C patients [94,95]. Consistent with the treatment strategy for KD, it is recommended to administer low-dose aspirin (3 to 5 mg/kg/day) as an antiplatelet agent for MIS-C patients (Table 3) [68,87,89]. This aspirin regimen is typically continued for a duration of four to six weeks or until coronary arterial dilation normalizes [68]. Moreover, in instances where the coronary arterial z-score reaches 10.0 or higher or when patients exhibit moderate to severe left ventricular dysfunction (EF < 35%), anticoagulation therapy is advised [96]. The benefit of anticoagulation for children with MIS-C, who lack large coronary arterial aneurysms or moderate to severe ventricular dysfunction, remains uncertain. Thus, treatment should be decided case by case, considering each patient's individual risk for thrombosis and bleeding [89]. The therapeutic approach to coronary artery ectasia and aneurysms adheres to the established KD guidelines [46,68].
Conclusion
MIS-C, a rare post-viral inflammatory disease associated with COVID-19, frequently presents with cardiovascular complications. The incidence rate and clinical manifestations of MIS-C have been affected by variant changes during the COVID-19 pandemic. However, common cardiovascular complications, including ventricular dysfunction, coronary arterial aneurysm, and conduction abnormalities, can be effectively managed via appropriate supportive care and immunomodulatory therapy. The trajectory of future research is anticipated to improve the differential diagnosis and treatment of evolving MIS-C cases, thereby fostering enhanced patient outcomes and enriching our understanding of this medical condition.